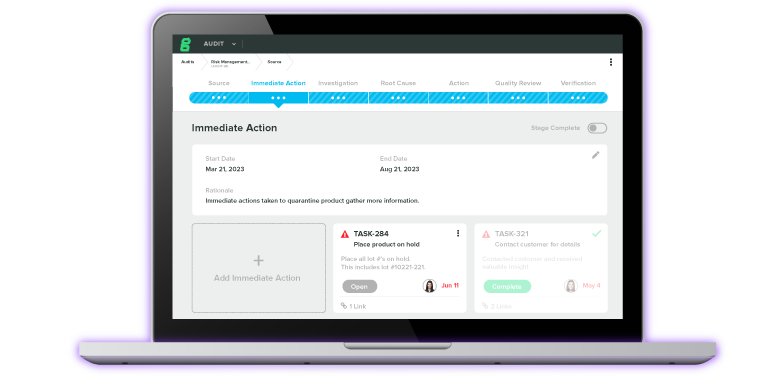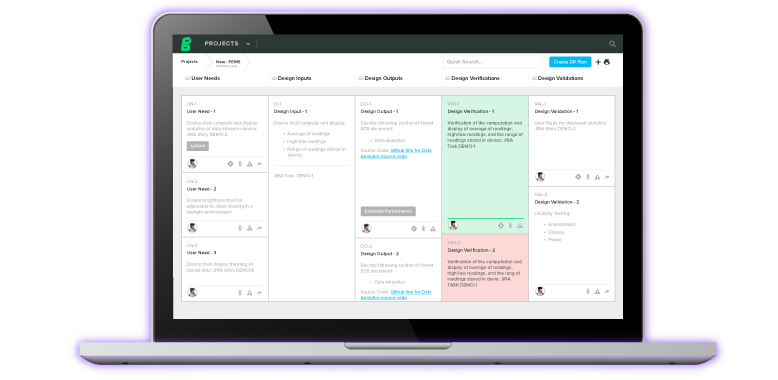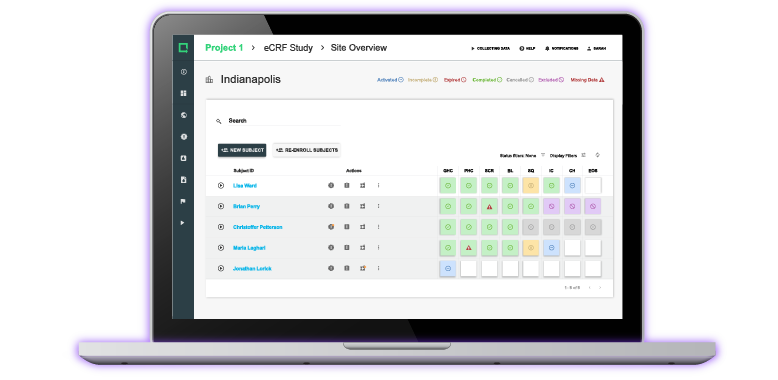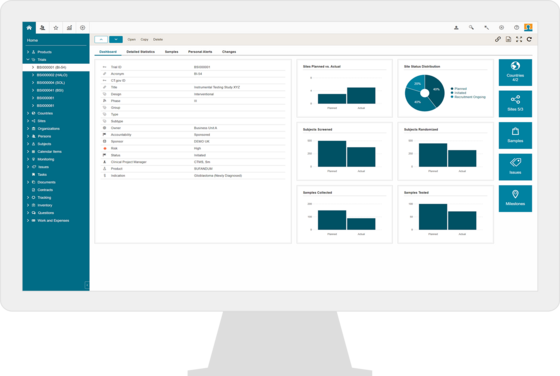
CTMS & eTMF for Clinical Investigations
Combine Greenlight Guru Clinical with CTMS and eTMF from BSI Life Sciences for a complete eClinical suite for MedTech clinical operations.
Plan, track and monitor all your pre- and post-market studies with complete oversight of all study relevant data in one system due to the seamless integration with Greenlight Guru Clinical.

Purpose Fit Add-ons for MedTech Clinical Operations
Streamline your clinical data collection and management with these simple, yet powerful add-ons.
ISO 14155:2020 & MDR compliant AE reporting fully integrated in the eCRF.
- Customise to fit your needs
- Flexible access & permissions
- Automatic notification to users & sponsors
- Real time overview and event progress tracking
- AE specific data export
ISO 14155:2020 & MDR compliant AE reporting fully integrated in the eCRF.
- Customise to fit your needs
- Flexible access & permissions
- Automatic notification to users & sponsors
- Real time overview and event progress tracking
- AE specific data export
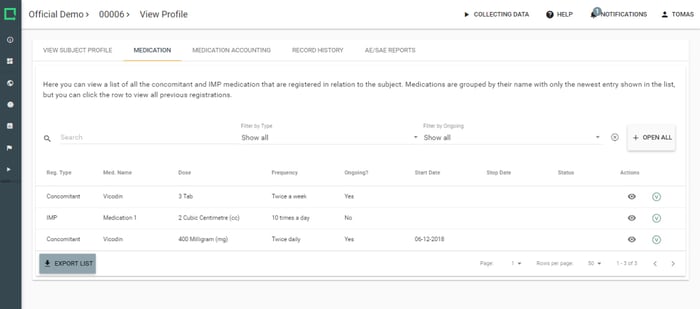
Collect vital information on concomitant medications and even link them with your Study AE/SAE reports.
- Standardise reporting on concomitant medication
- Standardise reporting on IMP
- Maintain an overview with medication accounting
Collect vital information on concomitant medications and even link them with your Study AE/SAE reports.
- Standardise reporting on concomitant medication
- Standardise reporting on IMP
- Maintain an overview with medication accounting
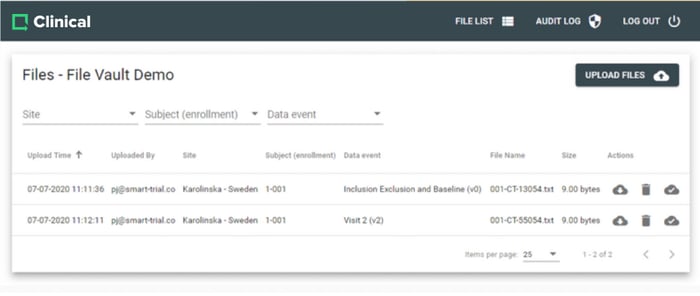
Collect and manage large files from your study with the Greenlight Guru Clinical file vault. A secure way to store large files such as MRI scans, CT scans, EKG recordings and more.
File Vault is fully integrated with Greenlight Guru Clinical and enables you to quickly collect and organise these files in your study.
Collect and manage large files from your study with the Greenlight Guru Clinical file vault. A secure way to store large files such as MRI scans, CT scans, EKG recordings and more.
File Vault is fully integrated with Greenlight Guru Clinical and enables you to quickly collect and organise these files in your study.
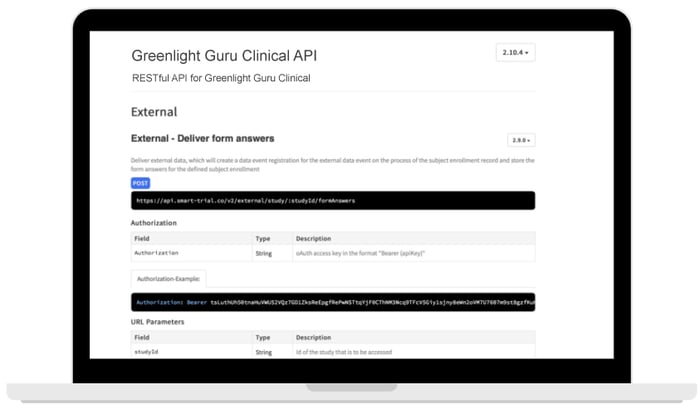
The Greenlight Guru Clinical API supports two way communication to push and pull data directly to/from your study. Hassle free, secure and fast transfer of any data into a Greenlight Guru Clinical study of your choice.
- Supports multiple devices
- Integrates with 3rd party applications
- Data export for internal storage
The Greenlight Guru Clinical API supports two way communication to push and pull data directly to/from your study. Hassle free, secure and fast transfer of any data into a Greenlight Guru Clinical study of your choice.
- Supports multiple devices
- Integrates with 3rd party applications
- Data export for internal storage
Greenlight Guru Clinical provides block randomization with variated block sizes. It is simple to set up and use, fulfilling all quality requirements that are relevant for this particular function.
Greenlight Guru Clinical provides block randomization with variated block sizes. It is simple to set up and use, fulfilling all quality requirements that are relevant for this particular function.



Add-ons That Will Make Your Subjects and Site Teams Happier
These features aim to enhance the experience of subjects and boost their rates of participation in your studies.
Regulatory-compliant and subject-friendly method that allows subjects to sign informed consent digitally.
- Comply with 21 CFR part 11 and EU standards for electronic signature
- eSignature methods based on location preferences
Regulatory-compliant and subject-friendly method that allows subjects to sign informed consent digitally.
- Comply with 21 CFR part 11 and EU standards for electronic signature
- eSignature methods based on location preferences
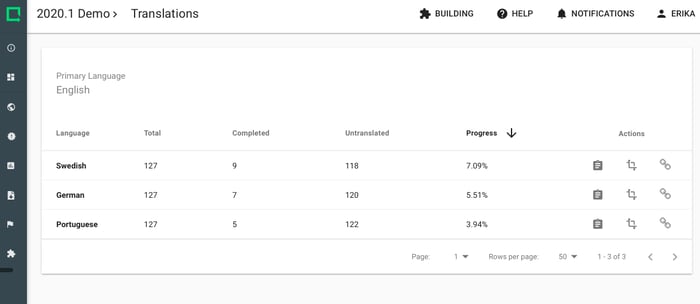
Let subjects fill out questionnaires in their own language. All data is stored in the primary language and can be exported with the primary Study language that you choose.
Let subjects fill out questionnaires in their own language. All data is stored in the primary language and can be exported with the primary Study language that you choose.
Improve your productivity with study notifications. Use the prompts to go into the system only when your attention and action is required.
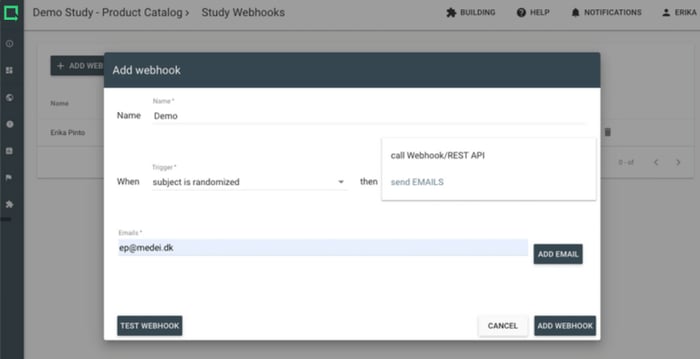
Improve your productivity with study notifications. Use the prompts to go into the system only when your attention and action is required.
Add your custom Logo to Greenlight Guru Clinical.
Add your custom Logo to Greenlight Guru Clinical.
Enable SMS send-oust for quick responses from subjects and increase your response rates.

Enable SMS send-oust for quick responses from subjects and increase your response rates.