How Technology Can Support Clinical Trial Continuity & Data Integrity
.png)
The Covid Pandemic impacted the way we live and work. Its impact was felt on every aspect of what we did and no industry was immune. Naturally, that means that the MedTech industry was also affected immensely during 2020 and 2022 when Covid spread across the globe.
These unprecedented times required us to adapt our existing procedures with caution. The challenge many device companies faced was maintaining the continuity of work which in large part depended on participation by subjects, and to navigate this while complying with regulations and ensuring the safety of staff and subjects. This was not an easy task as the problem was novel to all.
The FDA and the EU have several considerations to guide sponsors and investigators through pandemic-like situations. However, the common thread in these guidelines is the focus on safety of participants, maintenance of Good Clinical Practice, minimizing risk to trial & data integrity and documenting reasons for protocol deviation.
To help address these challenges, many are looking to technology for help. As the major impacts of the pandemic wane, this trend toward technology in clinical studies continues. Below, we have listed a few ways to leverage modern technology to keep trials running..
Protocol Deviations as a result of skipped/missed visits
Protocol deviations include missed or rescheduled visits in studies and should be documented when they occur. Deviations typically do not entail an increased risk for the patient but might affect the study outcomes.
To deal with protocol deviations, study teams should plan ahead by including a Protocol Deviation Form as a part of their Case Report Form. However, in cases where this has not been done, an amendment to the eCRF might be necessary.
The Greenlight Guru Clinical EDC system can support studies with its unscheduled event functionality. Users can include the Protocol Deviation Form as an “Optional Form” which can be activated on a subject level as an unscheduled event. This can be setup through the Process Creator.
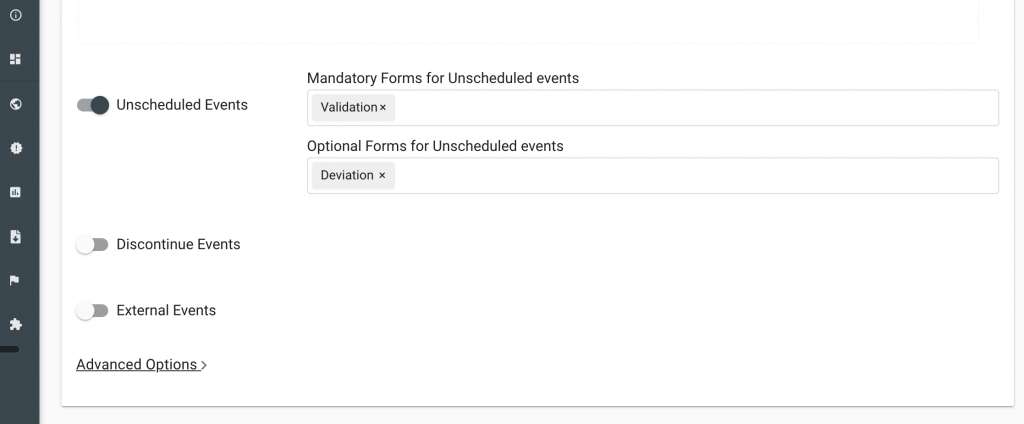 Add Deviation Form as an Unscheduled Event
Add Deviation Form as an Unscheduled Event
In cases where studies have not included a Protocol Deviation Form as a part of their initial study set up, it might be necessary to conduct a Study Amendment. These can easily be done within a matter of minutes and will not affect other data collection in the study.
Once a Protocol Deviation Form has been created (either in this study or saved to your profile) it can be added to the Unscheduled Event Optional Form section. Study Amendments are initiated by clicking the Study Amendment Button in the bottom left corner of the Study Setting Screen.
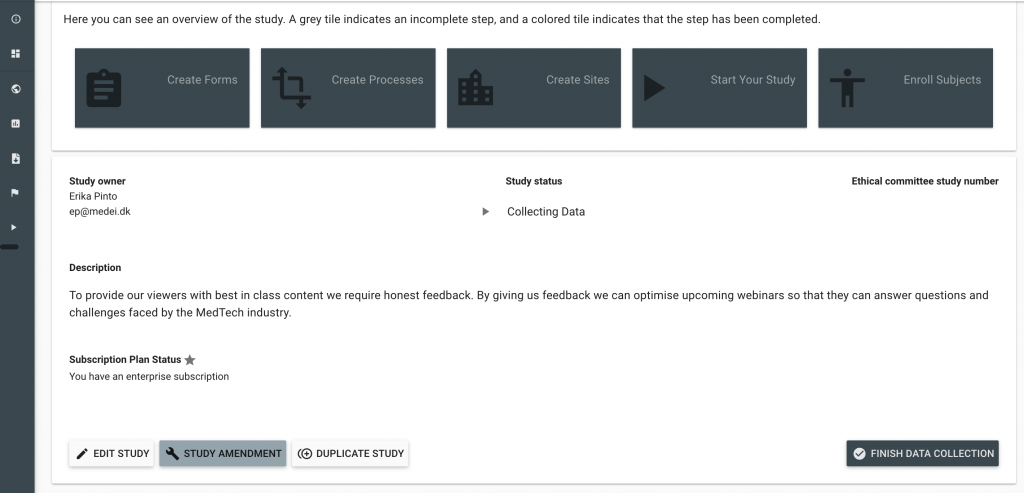 Amend an Active Study
Amend an Active Study
Keep patients engaged with electronic Patient-Reported Outcomes
During COVID-19, many patients were either unable or unwilling to travel to sites for scheduled visits or sites had to close due to social distancing measures. The challenge then was how to capture this data that was intended to be collected from study participants during in-person visits to study sites. To keep the clinical trial running, medical device manufacturers must keep participants engaged.
To accommodate for the lack of on-site visits and missing data, sponsors should evaluate the use of electronic patient-reported outcomes (ePRO). ePRO can be of great benefit to otherwise missing data directly from subjects remotely.
Greenlight Guru Clinical provides a simple way to send questionnaires to subjects directly to their email or mobile phone via SMS, where subjects can answer the questionnaires at home or on the go.
This works on most modern computers and smart devices like tablets and smartphones. Data is sent directly to the Study and stored securely in compliance with regulations and industry standards.
We are seeing our clients make full use of the Greenlight Guru Clinical ePRO capabilities for subjects that are enrolled to answer questionnaires on their own smartphone. This allows them to go on capturing data. They are then opening deviation forms for visits that were scheduled and are now phone calls.
- BYOD - Send participants surveys/questionnaires and allow them to fill them out on their own device/smartphone
- Consider using Phone calls and video calls as much as possible as an alternative
Keep patients informed
In times of crisis, it’s important to keep study participants informed of the situation that impacts their study participation and treatment. With the COVID-19 pandemic, many felt confused about how their participation would be affected, if at all, and how. It’s important to remember to share information with subjects on a regular basis to keep them engaged.
Subject (patient) engagement is easy to set up in Greenlight Guru Clinical. By setting up Information Events in a Study, a study manager can send emails and/or SMS to individual subjects, or all subjects, with relevant information and content on their study participation.
 Send emails from Greenlight Guru Clinical to participants & keep them informed
Send emails from Greenlight Guru Clinical to participants & keep them informed
Survey
As a medical device manufacturer, it is always a good idea to reach out to clinical professionals to gather information and feedback on your products. This can be helpful especially regarding design of a Post-Market Clinical Follow-Up plan.
One way to approach this for example is through Surveys, where Greenlight Guru Clinical’s EDC software can be used to send questionnaires to clinical professionals to answer a set of questions on their use of a device, or to provide case-by-case information on the device’s clinical performance.
Depending on the local legislative environment and if designed correctly, this might offer medical device manufacturers a chance to gather clinical data for PMCF use, without having to go through ethical approvals.
Greenlight Guru Clinical is centered around subjects, and a subject can be a patient, clinician, or any other participant. Thus, Greenlight Guru Clinical's ePRO can and has been utilized for much broader data collection than just PROs. It is flexible and can be set up in different ways based on your study requirements.
When Site visits are not allowed or prevented (Monitoring)
Leverage Verification I & II
Pandemics, weather conditions, canceled flights etc. can prevent monitors from making their planned on-site visits. Being prevented from visiting the site can force device companies to change their monitoring strategies to a remote based strategy.
Greenlight Guru Clinical offers two types of monitoring/verification features (Verification I and II) that can be used for a variety of things such as Source Data Verification (SDV), remote monitoring, site monitoring ,etc.
The features can be configured from the study setup menu and both have specific permissions that enable full control of who can do what with which Verification functionality.
These features can enable you to mark variables and reports that you have remotely monitored for data consistency, validity, etc. Furthermore, if you can get remote access to hospital information systems you can use the verification functionality to do remote SDV.

Remote enrollment with Informed Consent (eConsent)
One of the major challenges is enrolling participants into trials with informed consent when current consent methods are not feasible. Additionally, many companies are going back to the drawing board to map how participants are enrolled.
While technology cannot resolve all issues, it can be used in certain areas of the pipeline to patch problems. Authorities have recommended that any “validated and secure electronic system already used in the trial for obtaining informed consent can be used as per usual practice and if in compliance with national legislation”.
Greenlight Guru Clinical offers an e-consent integration to streamline the informed consent process, contact us to implement this in your studies.
What do we do and how can we help?
From our inception, Greenlight Guru's electronic data capture has been committed to the MedTech industry. We stand behind our vision for all MedTech companies to collect high-quality clinical data.
While these unprecedented times have and continue to have an impact on business, Greenlight Guru Clinical remains fully committed to serving the Industry and professionals that rely on us the best we can.
Ready to Learn More? Contact us for a Customized Demo.
Páll Jóhannesson, M.Sc. in Medical Market Access, is the founder and Managing Director of Greenlight Guru Clinical (formerly SMART-TRIAL). Páll was previously the CEO of Greenlight Guru Clinical where he led the team to create the only EDC specifically made for medical devices.










