Superior Ad-Hoc Data Capture
Collecting GCP compliant ad-hoc data has been a tough nut to crack for many medical device manufacturers – Greenlight Guru Clinical's Cases module is here to fix that. Empower clinical staff to easily deliver data either during or after application of a medical device in practice, and ensure fresh-in-mind data while minimizing their burden.

Simple and Compliant Prospective Data Collection
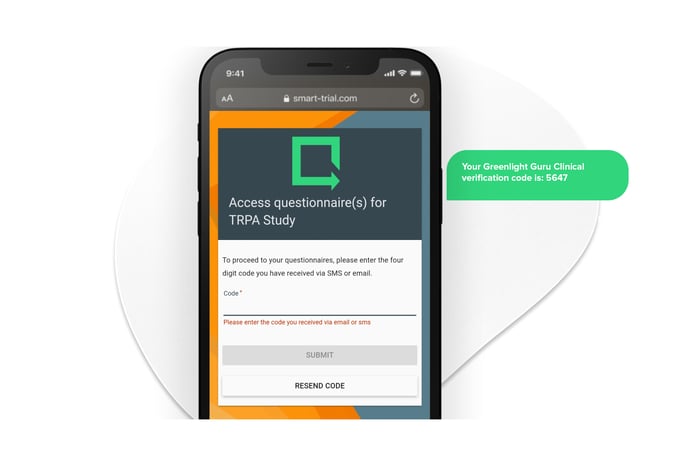
Greenlight Guru Clinical Cases is specifically designed to enable device manufacturers to collect high quality and compliant ad-hoc prospective data in post-market settings.
Simplified Post-Market Ad-Hoc Data Collection
Collect clinical outcomes, safety & vigilance, clinical experience, and usability data with ease.
Fast and intuitive 3-step study builder. 90 seconds is all it takes!
Fast and intuitive 3-step study builder. 90 seconds is all it takes!
Adapt forms according to different devices or product families and keep track of all post-market data on products within the same family – all in one place. No need to create projects or studies for each device.
Adapt forms according to different devices or product families and keep track of all post-market data on products within the same family – all in one place. No need to create projects or studies for each device.
Distribute each study however you prefer, you can even print a QR-code directly on your device packaging.
Distribute each study however you prefer, you can even print a QR-code directly on your device packaging.