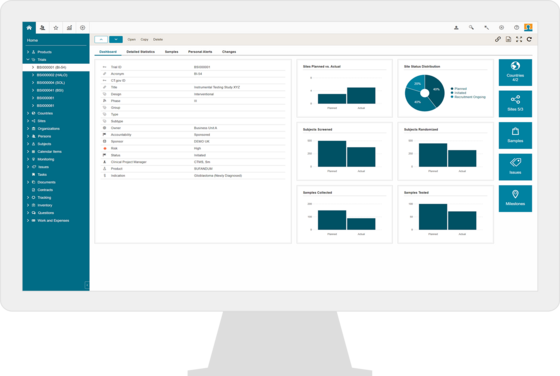
Clinical Trial Management System (CTMS)
BSI CTMS is the backbone of your future clinical trial management. Plan, track and monitor all your pre- and post-market studies with complete oversight of all study relevant data in one system due to the seamless integration with Greenlight Guru Clinical.
- Study design and study setup based on templates
- Local, regional and global studies
- Site and principal investigator (PI) data base
- Study and site budgets
- Milestones and activities
- Risk based site monitoring
- Offline monitoring
- Tracking of risks, issues, protocol deviations and (SAEs)
- Risk based site contract and invoice tracking
- Tracking of complete study communication (e-mail, phone, calls, letters...)

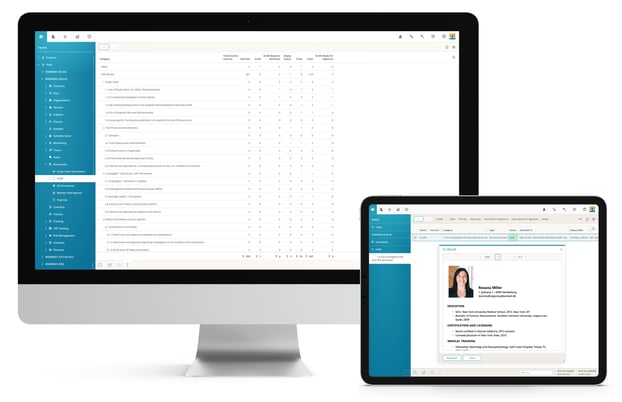
Electronic Trial Master File (eTMF)
BSI eTMF delivers comprehensive functionality for all aspects of trial master file management for your clinical trials from study startup, to execution, flexible reporting and closing.
Full support of your own TMF structure or the TMF Reference Model from DIA with controlled access for all study partners e.g. sponsors, CRO and sites. Setup document plans and track the creation, reviewing and approval process with enhanced alerting functions. Perform regular quality control of all study documents.
- TMF reference model from DIA
- Document plans with automatic creation of placeholders
- Work on, upload, link, and save documents and e-mails
- Review and approval workflows including electronic signatures
- Document deadlines with alerting functions
- Download complete eTMF with optional PDF/A conversion










